Insights: Hello, Benoit. How is treating the brain a challenge for science? What are the unique features of this organ that make administering therapy so complex?
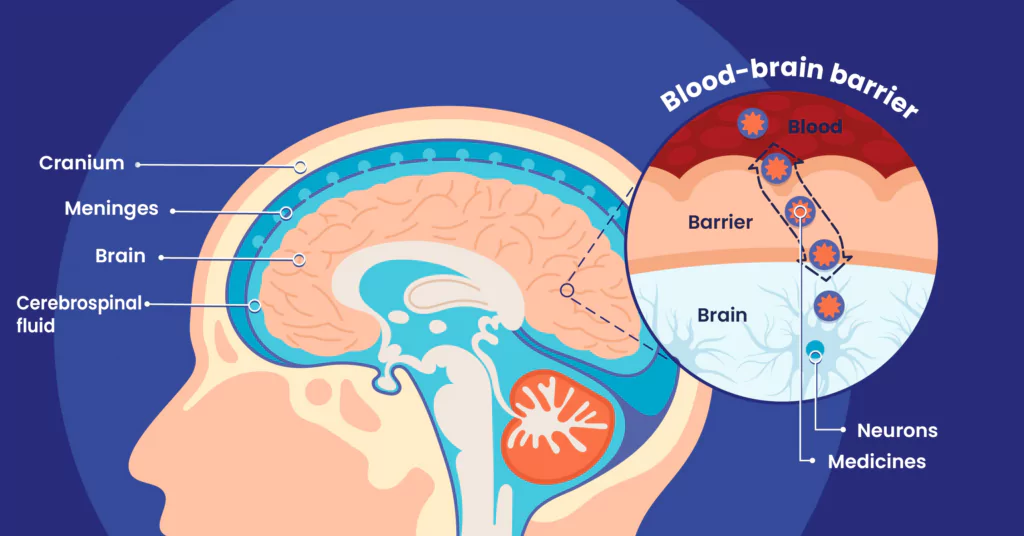
Benoit Larrat: The brain is unique in that it is an organ that is extremely well-protected from the outside, both mechanically by a solid braincase, and physiologically, by blood exchange systems that are extremely regulated with the extracerebral medium, the ventricles — that is to say cerebrospinal fluid (CSF) — or blood.
The most selective interface is the blood-brain barrier (BBB). Its role is to regulate exchanges between the blood and the brain, and particularly to prevent the pathogens that are potentially present in the bloodstream from entering the brain. The blood vessels found in the brain are distinctive in that they are very tightly sealed. The endothelial cells that compose them are linked together by ‘tight’ junctions, which are protein junctions that prevent most substances — whether harmful or therapeutic — from passing through.
For almost all extracerebral molecules, it is nearly impossible to pass through this barrier. It is estimated that more than 95%1 of known pharmaceutical molecules cannot make it through to the brain!
Of course, for the brain, the nature of such a protective system is vital from the outset because this protects it from harmful elements. It also helps to ensure stable biochemical conditions in the brain. This is called homeostasis.
Nevertheless, when it comes to treating a patient with a brain disease, such as a tumor or neurodegenerative disease, it is a real challenge to administer the therapeutic molecule to reach targets located in the intracerebral environment. In fact, although I am convinced that molecules exist today to treat most of these pathologies, we are not able to get sufficient quantities of them to the areas they need to treat.
Benoit Larrat

Benoit Larrat is a graduate of ESPCI Paris in Physics and holds a doctorate in Medical Instrumentation. He did his post-doctorate at NeuroSpin on ultrasound as a delivery route of substances to the brain. A tenured CEA research engineer since 2012, he developed this technology in animals by collaborating with several other academic and industrial laboratories. In 2020, the TheraSonic project integrated the CEA’s technological maturation and spin-off program. Together with his co-founder Anthony Novell, a researcher at CNRS, he designed and developed a prototype medical device based on previous work. At the end of 2023, TheraSonic officially launched and carried out its first fundraising campaign. The start-up’s objective is to validate this technology as quickly as possible on the first patients in neuro-oncology, then to establish pharmaceutical partnerships in order to demonstrate the added value of the approach combining innovative biotherapies and ultrasound.
The brain is physically and physiologically protected by several protective barriers, each of which plays a very specific role. These include:
- Cranial bones
- Meninges
- The mechanisms regulating exchanges between cerebrospinal fluid-filled cerebral ventricles and the brain
- The blood-brain barrier (BBB)
Insights: And so, where does research stand in this area today? What solutions exist to reach the brain, and ultimately, deliver a therapeutic substance within it?
B. L: : I would say that currently, the goal is to be able to deliver the right dose of medicine to the right therapeutic target without having to resort to using delivery technology. Caring for the brain also means balancing the benefit-risk for the patient, finding the right compromise between the quantity of medicine sufficient and the toxicity induced by treatment. Given the small proportion that accesses the brain, especially for the most promising biotherapies, the peripheral toxicity is what limits the total acceptable dose to be administered to the patient. We must not risk administering a dose that is too high. Much of the effort therefore consists in increasing active ingredients’ natural capacity to cross the BBB while limiting their peripheral side effects.
Caring for the brain
also means balancing
the benefit-risk for the
patient.
Then, a second point that researchers are actively examining is solving the problem by offering patients new methods of administration, which is our case.
Today, several methods exist to reach brain tissue. These include invasive intracerebral delivery by needle, convection-enhanced delivery (CED), direct injection into cerebrospinal fluid, intranasal nebulization of active ingredients, implants loaded with therapeutic agents, etc. These technologies show some effectiveness but cannot always be implemented. In addition, they suffer from limitations such as invasiveness or lack of targeting. For our part, we have based our approach on transcranial ultrasound technology.
Insights: Tell us about your technology. How does it work? How could ultrasound allow a therapeutic molecule to reach its target?
B. L: Our technology is a drug delivery system, like the systems discussed above. Its purpose is to transport therapeutic substances to a target located in the brain.
Our approach consists of emitting ultrasound in a pulsed manner, which will increase the permeability of the blood-brain barrier locally for a time (T) long enough to allow the therapeutic substance to be administered to the patient.
In very concrete terms, the patient receives microbubbles of air intravenously, which are already used as contrast agents for ultrasound. These bubbles will oscillate under the effect of ultrasound. In contact with the walls of the cerebral blood capillaries, they will generate stress inside the vessels, which will induce a temporary opening of the tight junctions. Ultimately, this allows therapeutic substances a much better passage in this location. Depending on the type of medicine, brain concentration can be increased 2 to 20-fold compared to normal.
The field of application is vast. Our technology can transport all classes of therapeutic molecules to the brain: small molecules, nanoparticles, therapeutic and targeted antibodies, as well as viral vectors for gene therapies, with the objective of treating neurodegenerative or rare diseases, cancers, or psychiatric diseases.
Insights: What exactly does your technology bring to this field and how is it beneficial for the patient?
The first advantage of an ultrasound approach, and the most important criterion for us, is that it is non-invasive for the patient and offers a simpler care pathway. Indeed, after extensive discussions with health care professionals, many raised the point that patients were reluctant to proceed with treatments that were too invasive both physically and mentally (the duration of the procedure, or even the fact of having to shave their head!). This is key to getting patients to adhere to these treatments.
Our technology therefore takes place in outpatient medicine: one hour is enough for the patient to receive their treatment via ultrasound.
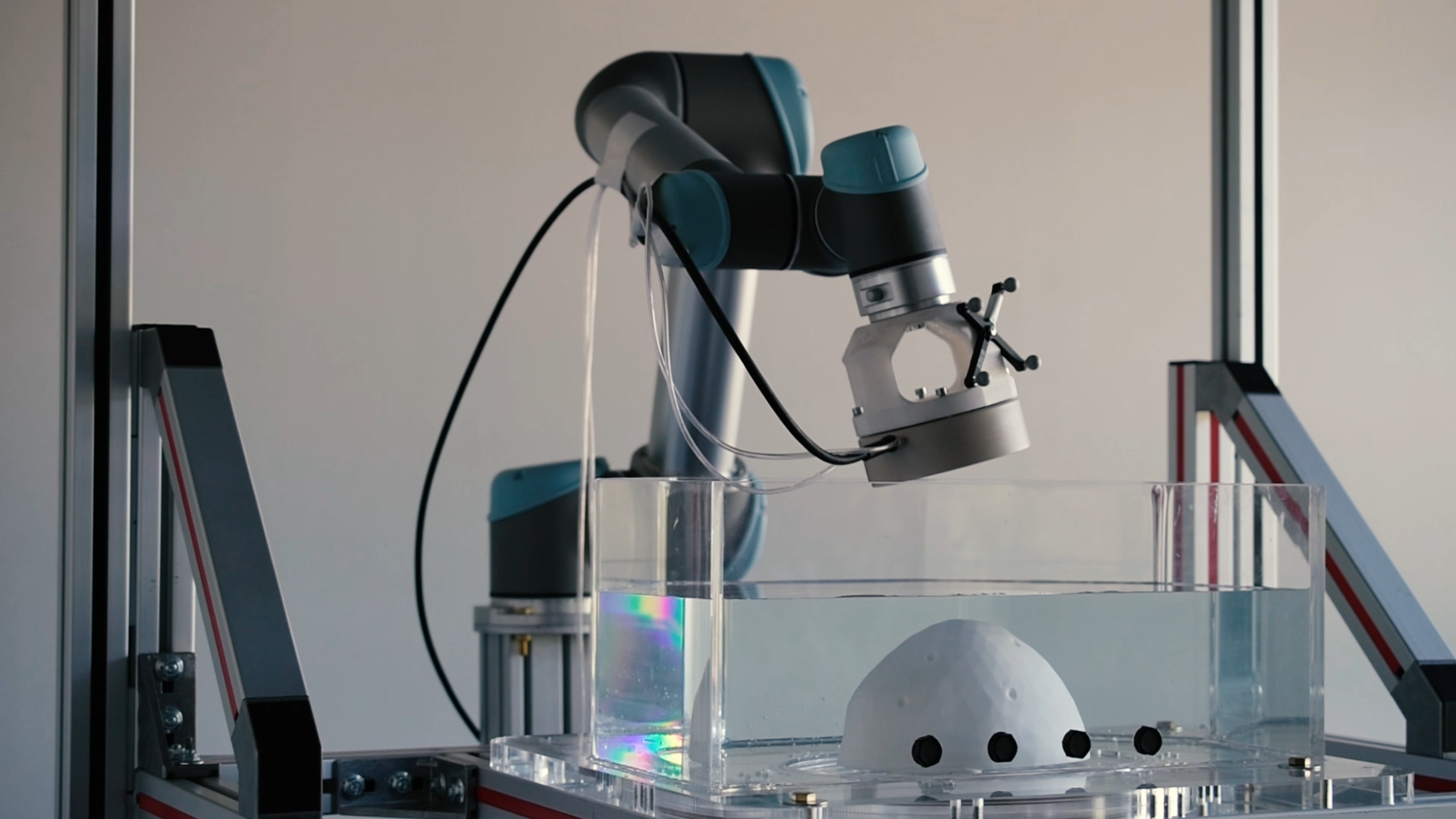
Next, the helmet we are developing is equipped with a dosimeter, which adapts the intensity of the treatment to the patient’s skull thickness and each treatment point. This precise approach will help better calibrate the dose of therapeutic substance administered to the patient, and ultimately deliver the most appropriate treatment for both adults and children.
Finally, to aim accurately and treat multiple brain volumes automatically, we rely on a robotic arm that precisely controls the ultrasound beam to scan the areas to be treated, which are determined by physicians according to the patient’s medical images.
Insights: What are the next steps in developing this solution? In other words, how soon can patients with brain diseases expect to benefit from this medical approach?
B. L: Today, we are still in the pre-clinical phases, and we hope to be able to submit a dossier to set up a clinical study in 2025. To this end, we have just finalized an initial fundraising campaign of nearly one million euros from the venture studio M2care and Gustave Roussy Transfert.
We are fortunate to be working within the Paris-Saclay cluster, an ecosystem that is particularly rich and stimulating for the development of innovations in the health care sector. We are convinced that as a start-up, collaboration with other partners is essential to the development of our technology. We are also stakeholders in the Paris-Saclay Cancer Cluster, an oncology cluster made up of public and private players from the Paris-Saclay geographical area. Its mission is to accelerate innovation in cancer treatment by providing an ideal framework (mentors, network, funding, clinicians, data, samples, technologies, infrastructures and laboratories) in which to develop innovative projects in the field of oncology.

Did you know?
As a committed player in the fight against cancer, Servier joined the Paris-Saclay Cancer Cluster (PSCC) in November 2023.
Claude Bertrand, Executive Vice President Research & Development, Chief Scientific Officer, sits on the Board of Directors, and several Group employees have also joined the biocluster’s scientific committees of governance or working groups to provide their expertise in the field of oncology.
[1] W. M. Pardridge, “Blood-brain barrier drug targeting: the future of brain drug development”, Mol Interv, vol. 3, 2003, p. 90–105 (PMID 14993430, read online [archive], viewed on May 16, 2011)